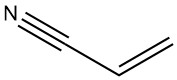

Acrylonitrile is a chemical compound with the chemical formula C3H3N. It is a clear, colorless liquid at room temperature and possesses a nitrile functional group attached to a carbon-carbon double bond. Acrylonitrile is an essential intermediate in the chemical industry and serves as a building block for various products.
It goes by various names, including 2-propenenitrile, propenenitrile, acrylic acid nitrile, propylene nitrile, vinyl cyanide, and propenoic acid nitrile.
The commercial synthesis of Acrylonitrile employs the propylene ammoxidation process, also known as the SOHIO process. This method, first introduced in 1960, involves the reaction of propylene, ammonia, and oxygen using a heterogeneous catalyst:
Before the discovery of the propylene ammoxidation process, acrylonitrile was predominantly produced through the ethylene cyanohydrin process.
The implementation of the SOHIO process brought about a significant reduction in the production cost of acrylonitrile, leading to a substantial surge in demand for this compound across a broad spectrum of chemical and polymer products.
Acrylic fiber remains the primary application for acrylonitrile, while markets for acrylonitrile-butadiene-styrene (ABS) resins, adiponitrile, and acrylamide have also experienced rapid growth. Consequently, the global annual production capacity for acrylonitrile now exceeds 5 millions t/a.
Acrylonitrile is a clear, colorless liquid when at room temperature. This molecule possesses a polar nature owing to the electronegative nitrile functional group, which is conjugated with a carbon-carbon double bond.
In terms of solubility, acrylonitrile demonstrates miscibility with several organic solvents, such as acetone, benzene, carbon tetrachloride, diethyl ether, ethyl acetate, ethylene cyanohydrin, petroleum ether, toluene, certain kerosenes, and methanol.
The following table lists some physical properties of acrylonitrile.
| Property | Value |
|---|---|
| Molar Mass | 53.064 g/mol |
| Density | 0.81 g/cm³ |
| Melting Point | -83.5 °C |
| Boiling Point | 77.3 °C |
| Refractive Index | 1.391 |
| Miscibility in Water | 7.30 wt% |
| Viscosity | 0.35 cP (at 20 °C) |
| Critical Pressure | 35.3 MPa |
| Critical Temperature | 245.8 °C |
| Autoignition Temperature | 481 °C |
The distinctive chemical reactivity of acrylonitrile originates from the presence of two reactive sites, namely the carbon-carbon double bond and the nitrile functional group.
The principal chemical reactions it undergoes are polymerization and hydration. Acrylonitrile readily and exothermically polymerizes in the absence of a hydroquinone inhibitor, especially under light exposure.
This polymerization process is initiated by free radicals, redox catalysts, or bases and can take place in the liquid, solid, or gas phase. Liquid-phase polymerization is particularly suitable for producing homopolymers and copolymers.
When acrylonitrile undergoes hydration with sulfuric acid, it forms acrylamide sulfate (C3H5NO·H2SO4), which can be converted to acrylamide through neutralization with a base and complete hydration leads to the formation of acrylic acid.
Acrylamide can also be directly generated from acrylonitrile via partial hydration with copper-based catalysts, which has become the preferred commercial route for acrylamide production.
Industrially important acrylic esters are formed by the reaction of acrylamide sulfate with organic alcohols.
The commercial production of methyl acrylate involves adding methanol to acrylamide sulfate.
Additionally, acrylonitrile engages in other reactions, including Diels-Alder addition to dienes, resulting in cyclic products.
Hydrogenation over metal catalysts yields propionitrile and propylamine. Moreover, a significant industrial reaction is the hydrodimerization of acrylonitrile, producing adiponitrile.
Furthermore, acrylonitrile can undergo halogen addition across the double bond to generate dihalopropionitriles. It also participates in cyanoethylation reactions with alcohols, aldehydes, esters, amides, nitriles, amines, sulfides, sulfones, and halides.
Acrylonitrile is industrially produced through a catalytic vapor-phase process called propylene ammoxidation, developed by SOHIO. The process employs a fluidized bed reactor in which propylene, ammonia, and air react with a solid catalyst at temperatures of 400-510 °C and pressures of 50-200 kPa gauge.
The reaction is single-pass, with approximately 98% conversion of propylene. Highly selective catalysts have led to lower propylene consumption, around 1.1 kg per kilogram of acrylonitrile produced.
Alongside acrylonitrile, the process yields useful coproducts such as HCN (used in methyl methacrylate and sodium cyanide manufacture) and acetonitrile (a valuable solvent in pharmaceutical and industrial applications).
In the commercial production of acrylonitrile, the hot reactor effluent is cooled with water in a countercurrent absorber, and unreacted ammonia is neutralized with sulfuric acid. The resulting ammonium sulfate is recovered and used as fertilizer.

The absorber off-gas, containing N2, CO, CO2, and unreacted propylene, is either vented directly or passed through an incinerator to combust hydrocarbons and CO. The acrylonitrile-containing solution is then processed in recovery columns to obtain crude acrylonitrile and crude acetonitrile, with further purification for acrylic fiber quality acrylonitrile.
The commercial viability of acrylonitrile production from propylene and ammonia was achieved in 1959 when SOHIO developed a catalyst with high selectivity for acrylonitrile. Improvements over the years have been mainly due to the development of new catalysts with increased acrylonitrile yields from propylene.
Multi-component mixed metal oxides, primarily based on bismuth-molybdenum oxide, have been the key catalysts, with various improvements achieved through the incorporation of iron, cobalt, nickel, and alkali metals.
Molybdate-based catalysts are predominant, but antimonate catalysts are also used commercially. Research efforts have focused on understanding the surface reaction chemistry and solid-state mechanisms of bismuth molybdate-based catalysts.
Kinetic studies have shown the rate-determining step involves the abstraction of a hydrogen atom from propylene to form a p-allyl complex on the surface, leading to the formation of acrolein and, ultimately, acrylonitrile.
Various techniques, such as Raman-spectroscopic analysis, X-ray, neutron diffraction, X-ray absorption spectroscopy, pulse-kinetic studies, and probe molecule investigations, have provided a deeper understanding of the complex solid-state and surface mechanisms involved in propylene ammoxidation over bismuth molybdate-based catalysts.
Acrylonitrile finds significant end uses in various industries, including acrylic fiber, acrylonitrile-butadiene-styrene (ABS) resins, adiponitrile, acrylamide, nitrile rubbers, and carbon fibers. Among these applications, acrylic fiber is the largest commercial use.
| Product | % |
|---|---|
| Acrylic fiber | 42 |
| ABS resins | 34 |
| Adiponitrile | 8 |
| Acrylamide | 7 |
| Nitrile rubber | 5 |
| Carbon fiber | 2 |
| Other | 2 |
However, ABS and acrylamide are expected to be the fastest-growing applications for acrylonitrile, with carbon fibers also on the rise, particularly in high-strength and lightweight applications within the airline and automobile industries.
Acrylic fiber is primarily utilized in the manufacturing of apparel and home furnishings. However, its market growth has slowed compared to other uses of acrylonitrile, with much of the acrylic fiber production capacity shifting from the U.S. and Europe to Asia, especially in China.
ABS, a specialty high-performance polymer, gains popularity due to its excellent strength, coloring properties, and ease of processing. It finds applications in various industries, including automotive, construction, appliances, and electronics. SAN copolymers, known for their optical transparency, are used in packaging, optical fibers, and food containers, among other applications.
Adiponitrile serves as a raw material for the production of hexamethylenediamine (HMDA, C6H16N2) through electrohydrodimerization. HMDA is then used to make nylon-6,6.
Acrylamide is manufactured from acrylonitrile through a copper-catalyzed process. Upon polymerization, it finds extensive use in wastewater treatment, oil production, mineral processing, and paper manufacture.
Nitrile rubbers, which are butadiene-acrylonitrile copolymers, are highly valued in the industry for their chemical, oil, and ozone resistance, along with excellent flexibility, stability, and heat resistance. They are commonly used in the production of gaskets, seals, hoses, belts, and electrical cable jackets.
Acrylonitrile poses significant toxicity risks if ingested, inhaled, or absorbed through the skin. In its liquid or concentrated vapor form, it is corrosive and can cause skin burns resembling second-degree burns.
Overexposure to its vapors can result in severe conjunctival and respiratory irritation, as well as symptoms such as headaches, nausea, vomiting, weakness, and dizziness.
Prolonged exposure may lead to drowsiness, seizures, hallucinations, loss of consciousness, and even death. The onset of these toxic effects can be delayed, occurring anywhere from a few minutes to several hours after exposure.
Furthermore, acrylonitrile is considered a suspected cancer hazard, with the risk of cancer depending on the level and duration of exposure. In vitro studies have shown weak mutagenic properties, while in vivo studies did not confirm this effect.
Studies on animals have revealed harmful effects on fetal development and reproduction when exposed to toxic levels of acrylonitrile. Embryotoxic and teratogenic effects have been observed in animals when their mothers were exposed to high doses.
Studies conducted on acrylonitrile workers in China reported higher-than-expected rates of reproductive effects and abnormal fetal development. However, the reliability of these studies has been questioned due to uncertainties regarding data collection methodology, chemical exposures, social and lifestyle influences, and inconsistencies with other information.
Epidemiological studies on the relationship between acrylonitrile exposure and specific tumors have not provided clear evidence.
While data on occupational levels of human exposure do not indicate a definitive correlation between acrylonitrile and cancer, it is still prudent to treat acrylonitrile as a potential carcinogen. Exposure levels should be minimized, and contact with liquid acrylonitrile should be avoided.
During combustion, acrylonitrile produces highly toxic byproducts, including hydrogen cyanide, nitrogen dioxide, and carbon monoxide.
To protect workers, the Occupational Safety and Health Administration in the United States regulates acrylonitrile as a cancer hazard (29 CFR 1910.1045). The Permissible Exposure Limit (PEL) is set at 2 ppm in air averaged over an 8-hour period (Time-Weighted Average – TWA).
The Ceiling Limit (CL) is 10 ppm averaged over a 15-minute period. The odor of acrylonitrile is a poor warning sign for exposure since its odor threshold is in the range of 13 to 20 ppm, which is well above both the PEL and CL.
Toxicity levels for acrylonitrile have been defined as follows: